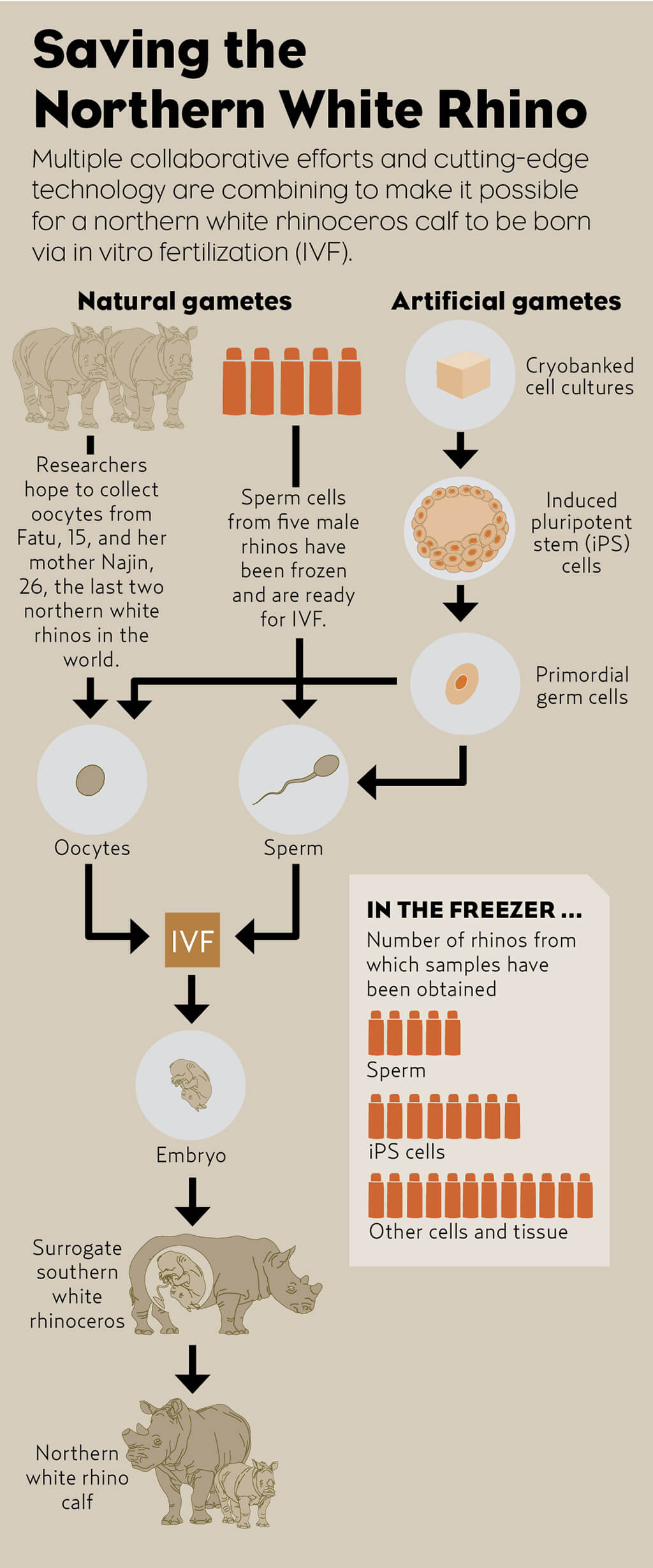
Stem Cells to the Rescue
By transforming skin cells into stem cells, San Diego Zoo Global is making important contributions to animal health, sustainable populations, and genetic rescue.
BY Oliver Ryder Ph. D.
I still want to pinch myself when I think that in our laboratory at the Beckman Center, adjacent to the Safari Park, we have made and are using stem cells to change the trajectory of species extinction.
Cells are life. They are the fundamental living unit, and only a cell can make another cell. Every living organism begins its life as a single cell or lives its entire life as one cell. DNA molecules in each cell of a plant or animal provide the program for its functions, including growth and development of all its tissues and parts. But DNA can only do this within a cell; it cannot make a cell on its own.

PRECIOUS CELLS
Oliver Ryder, Ph.D., Kleberg Endowed Director of Conservation Genetics at San Diego Zoo Global, examines DNA samples derived from northern white rhinoceros cells grown in the Frozen Zoo®. Conservation researchers are using samples for genomic studies and to facilitate crucial research for the northern white rhino genetic rescue initiative.
When I first learned about cells in school, I was amazed and wanted to know how it all worked. I am still amazed and still wondering to this day. Long ago now, I grew bacterial cells, initially for a science fair project, and then in my graduate studies. When I joined the conservation team at San Diego Zoo Global, I was excited to learn how to grow animal cells for the Frozen Zoo®, which was still in its infancy. Since then, conservation specialists have grown cells of literally thousands of animals of a multitude of species, and in doing so, learned a lot, honing their skills and bolstered by many important discoveries in the field.
Proof of the capabilities of these cells in the Frozen Zoo came dramatically to the fore when the 1996 birth of Dolly, the cloned sheep, came to light. An entire animal had been produced from a single adult cell, going through all the stages of development from embryo to lamb. The world was astounded, and no one more than I. The potential of the Frozen Zoo had been forever changed.

GONE BUT NOT FORGOTTEN
Cell lines derived from northern white rhinoceros and longtime Safari Park resident Angalifu are among those banked in the Frozen Zoo.
Yet another amazing discovery expanded the potential even further. By triggering the action of a small number of master genes, adult cells derived from a skin sample, such as those cryobanked in the Frozen Zoo, can be reprogrammed—rebooted, if you will—in the laboratory, to produce stem cells that are capable of becoming all the cell types of the body. This capacity, called pluripotency, is a characteristic of every successful embryo of a multicellular organism. The discovery of the method to induce or reboot cells to become pluripotent is one of the most important developments in the history of biological research.

NOLA AND HER CELLS
Northern white rhino skin cells (fibroblasts) have been added to the Frozen Zoo, beginning in 1979. Now, viable cells are cryobanked from 12 northern white rhino individuals, including the beloved Nola (at left).
We are poised to use that discovery in ways that benefit wildlife through medical applications and genetic rescue. Recognizing the potential of stem cells for regenerative medicine and animal health, our veterinarians are exploring treatments with stem cell technologies to improve the quality of life of wildlife in our care. We also hope to use this technology to prevent the extinction of species when all other methods fail.
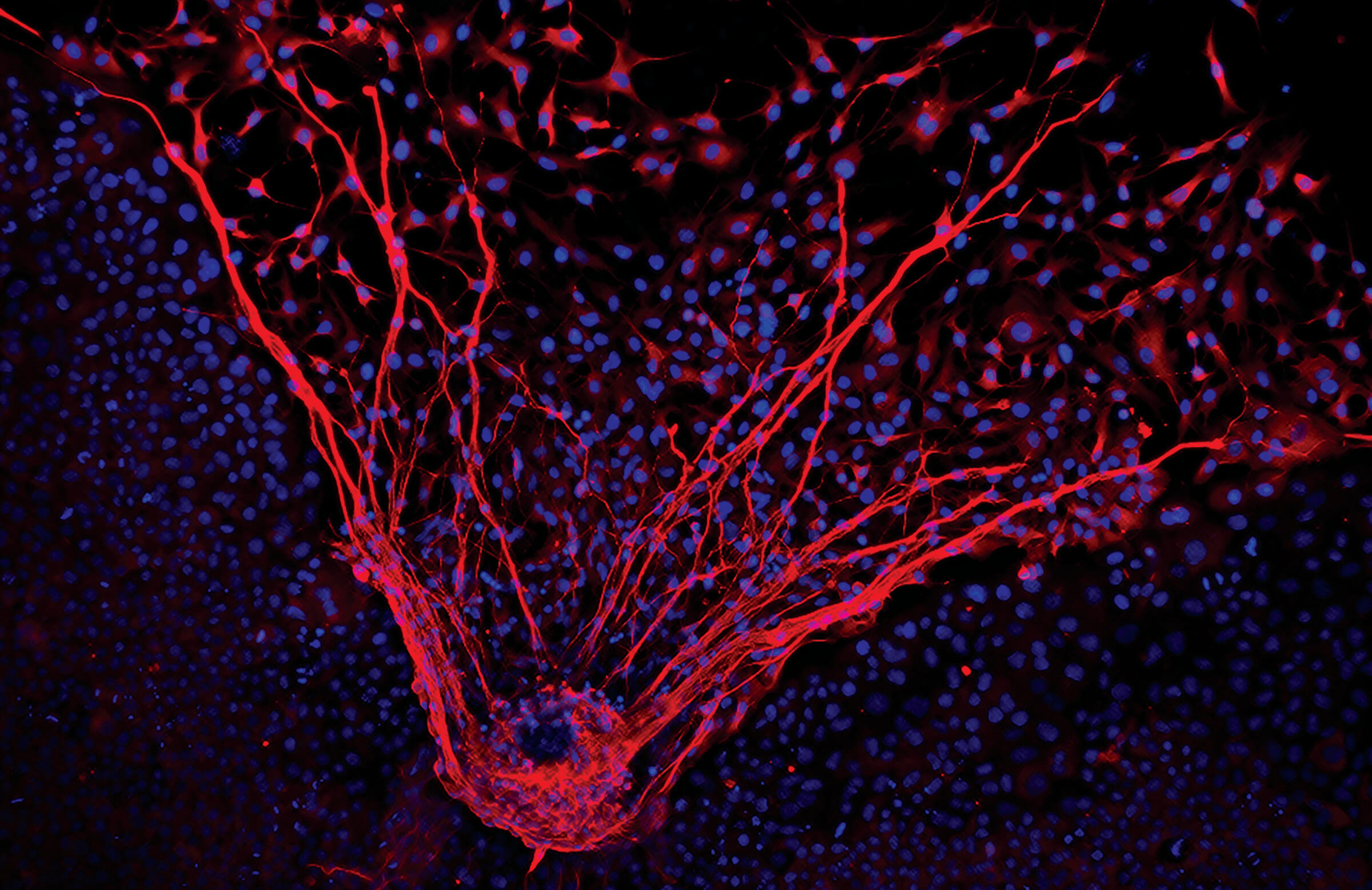
In the Stem Cell Laboratory, tucked away in a small corner of the Beckman Center, our scientists have taken cells from northern white rhinos, grown from skin samples collected and cryobanked in the Frozen Zoo more than 30 years ago, and produced induced pluripotent stem cells (iPSCs). We have characterized them in detail, and have demonstrated their ability to produce multiple cell types, such as neurons (brain cells, shown in top photo) and cardiomyocytes (heart muscle cells, shown in the video below).
HEART MUSCLE CELLS
San Diego Zoo Global wildlife researchers have demonstrated the ability to produce cardiomyocytes (heart muscle cells) from induced pluripotent stem cells (iPSCs).
The full potential of stem cells is a very active area of study, almost exclusively using model species such as the laboratory mouse—not animals that are under human care in zoos. Our efforts, unique and significant, are small in comparison with efforts to develop stem cell technology for regenerative medicine in humans. Still, we recognize that, although lab-based conservation science is taking place on a relatively small scale, it can have enormous impacts for species of concern.
We should all be tremendously encouraged by the potential of cellular and molecular technologies to prevent species extinctions. With some pride, we can say that this potential is closer to realization because of the many decades of investment and effort in cryobanking reproductive materials and lab-grown cells in the Frozen Zoo. This unique and precious resource enables our ongoing work to develop genetic rescue technology for a decidedly non-model organism, the northern white rhinoceros, for which only two females survive, mother and daughter.
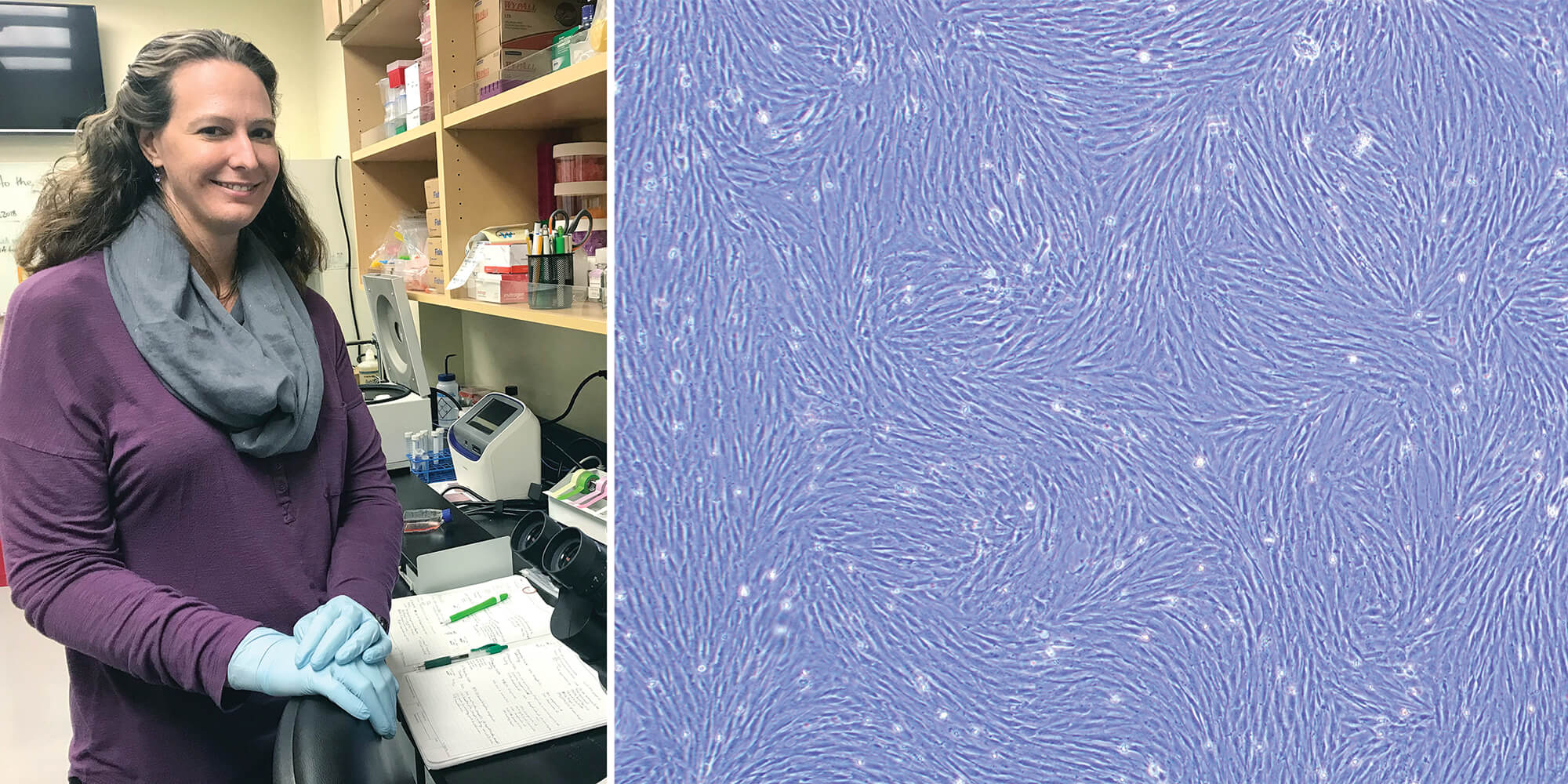
FROM SKIN CELLS TO STEM CELLS
(Left) Dr. Marisa Korody evaluates northern white rhino stem cells under the microscope. (Right) A microscopic view of northern white rhino skin cells (fibroblasts) growing in culture.
It is so evident that this effort needs to expand and take on a global scale. We draw satisfaction from the knowledge that what we are learning as we tackle the complex biology of mimicking development of a rhino embryo through stem cell culture in the laboratory can benefit other species of critically endangered rhinoceroses—and, we trust, other species—as our team enters areas of biological investigation never before explored.
Fulfillment of the ambitious goal to produce reproductive cells that can be used to produce embryos that develop into northern white rhino calves lies ahead, but we have taken the first, remarkable steps. Northern white rhino calves produced this way would not be clones; they would be unique individuals, produced literally as offspring of cells growing in our laboratory. Now, think again about the wonder that cells are!
Photography by: Ken Bohn/SDZG, Iñigo Valiente Alandi, and Marisa Korody.